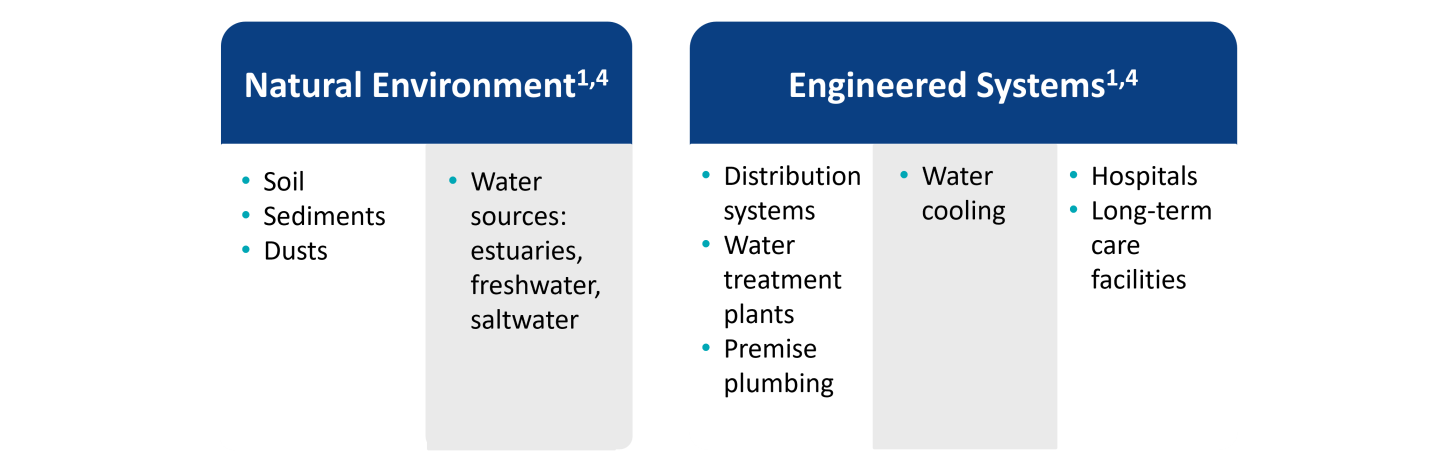
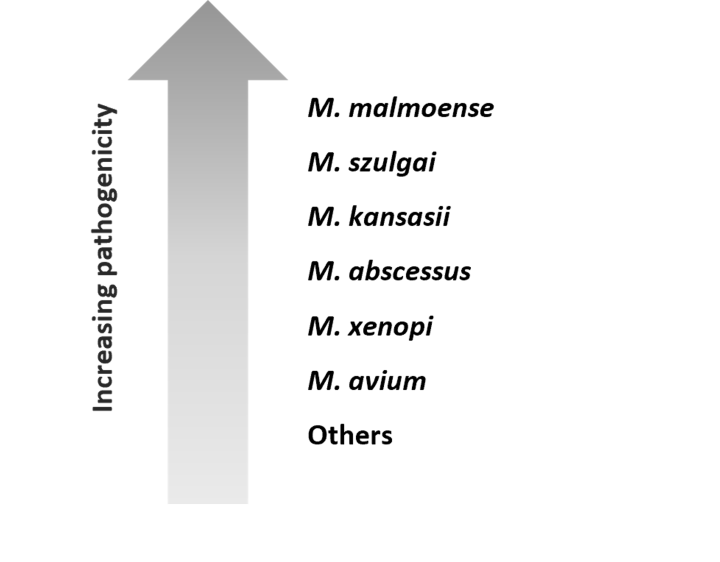
While exposure to NTM is common, the development of pulmonary disease is usually limited to individuals with predisposing risk factors including structural lung disease (bronchiectasis, chronic obstructive pulmonary disease, cystic fibrosis) or treatment with immunosuppressive medications (anti-TNFα therapy,a steroids).4
- Studies have demonstrated a close link between bronchiectasis and NTM pulmonary disease. Those with a reported diagnosis of bronchiectasis were 50 to 75 times more likely to have NTM infection compared with those without bronchiectasis1,2
- Cystic fibrosis is a particularly strong risk factor for NTM pulmonary disease, with a reported prevalence of between 6% and 13% in this group3
- In patients with rheumatoid arthritis, anti-TNFα therapy is associated with a significantly increased risk of NTM disease compared with non-users (adjusted odds ratio [OR]=2.19)4
Risk Factors for NTM Pulmonary Disease5 |
|---|
| Genetic | Cystic fibrosis
α-1-antitrypsin deficiency
Primary ciliary dyskinesia
Pulmonary alveolar proteinosis |
| Acquired | Bronchiectasis
Chronic obstructive pulmonary disease
Chronic aspiration
Lung malignancy |
| Lady Windermere Syndrome | Post menopausal females with slender body habitus and skeletal abnormalities |
| Drug induced | Anti-TNFα therapya,b
Cytotoxic therapya
Steroid therapy |
| Other | Vitamin D deficiency
Aspergillus infection |
aPatients on anti-TNFα therapy and cytotoxic therapy are predisposed to both NTM pulmonary disease and disseminated NTM infection, though lung disease is more common.
bTNFα, tumor necrosis factor alpha
Figure adapted from Ratnatunga CN, et al. Front Immunol. 2020 Mar 3;11:303 under the Creative Commons Attribution License (CC BY)5