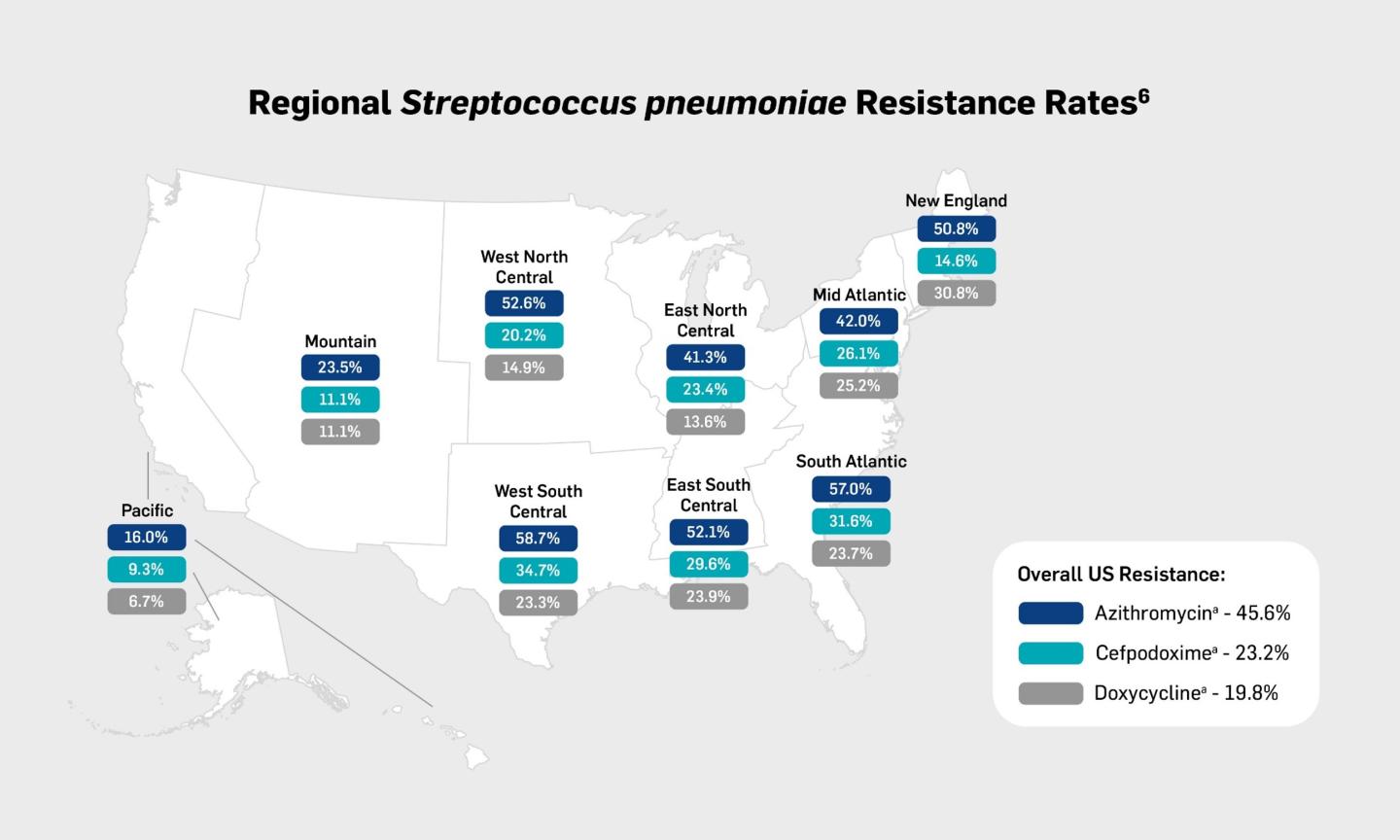
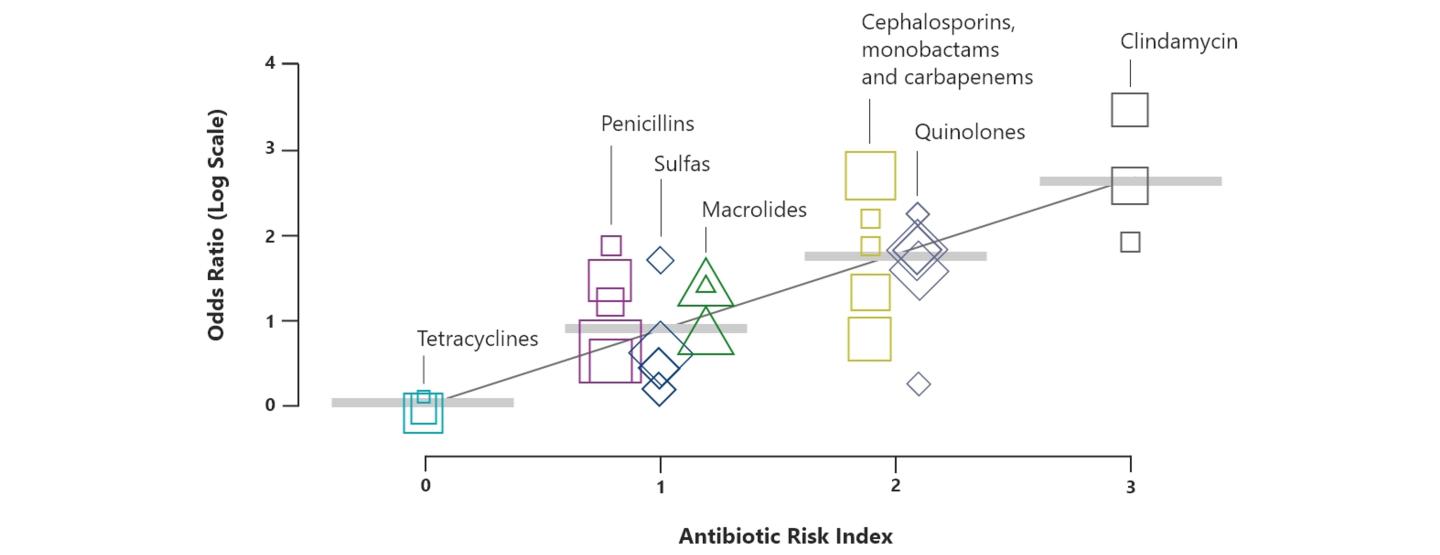
Antibiotic treatment failure contributes to the economic and humanistic burdens of CAP by increasing morbidity, mortality, and healthcare costs. The incidence of treatment failure in patients with CAP can range from approximately 10% to 20% of patients. Risk factors for treatment failure include older age (>65 years), high-risk pneumonia, liver disease, leukopenia, and discordant antimicrobial therapy.19 Inappropriate choice of empiric therapy has been linked to worse outcomes, including treatment failure, increased length of stay, hospital readmission, and mortality.20-22
Treatment failure and poor outcomes have been documented in CAP patients treated in the outpatient setting. A retrospective claims-based analysis of 251,947 adults with CAP treated as outpatients, found that 22% of patients failed antibiotic therapy, defined as any of the following within 30 days of initial antibiotic: refill of initial antibiotic, switch to a new antibiotic, emergency room visit for CAP, and/or hospitalization for CAP.22
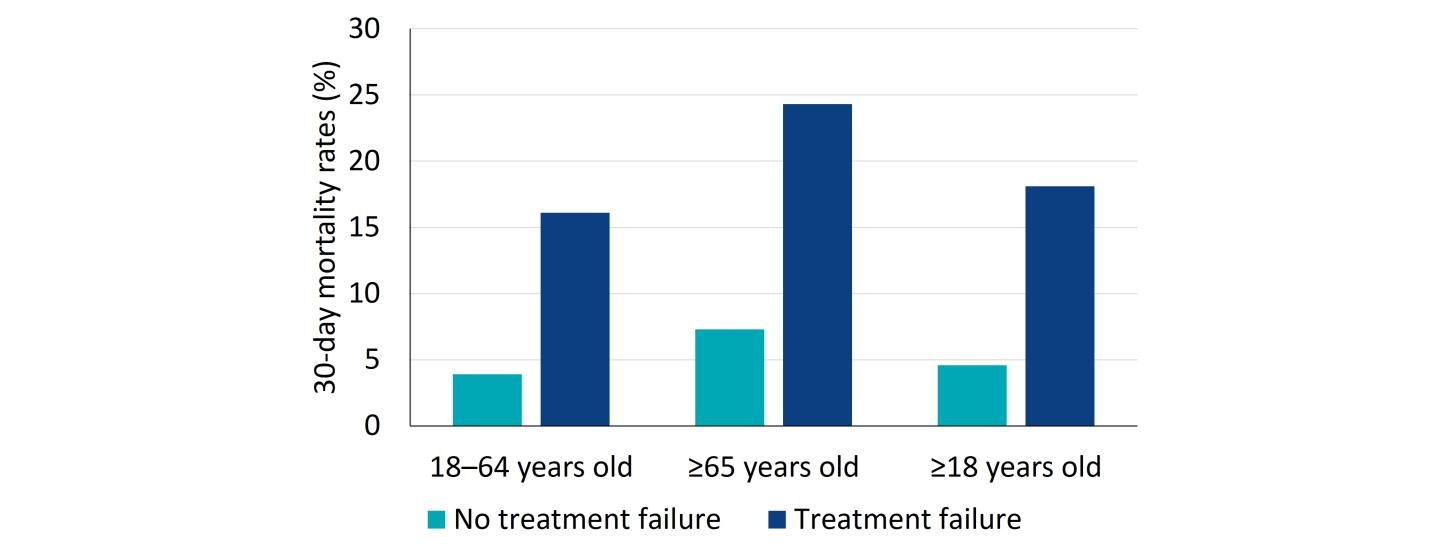
Patients with treatment failure also had higher all-cause mortality compared to patients with CAP who did not fail their antibiotic therapy (18.1% vs. 4.6%, respectively). The differences in 30-day mortality between antibiotic failure groups increased as a function of age.22 This study had several limitations. Diagnosis codes and pharmacy data were used to identify patients with CAP in the outpatient setting. Based on the nature of the data authors were unable to determine reasons for which patients received a new prescription, required a refill or subsequent outpatient or inpatient care. Based on the results of this study, the authors concluded that improvements in clinical management programs and therapeutic options are needed.22