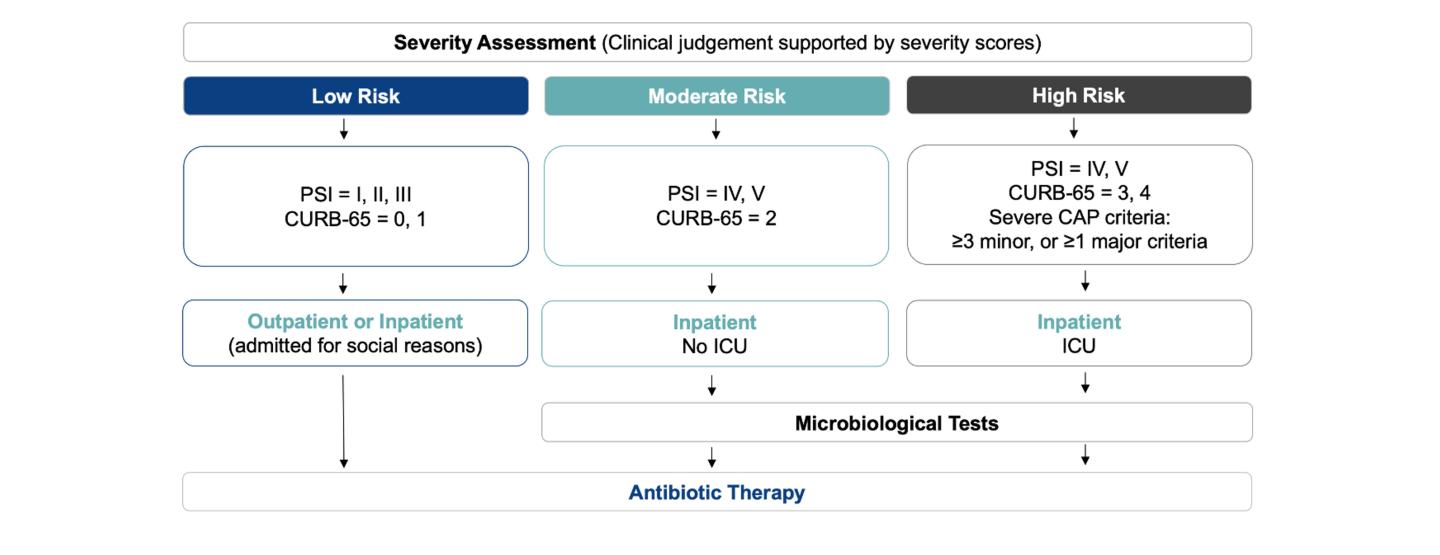
The ATS/IDSA guidelines suggest empiric treatment strategies as the standard of care, based on whether the patient is treated as an in- or out-patient, comorbidities, severity of CAP and risk factors for MRSA and Pseudomonas aeruginosa.1
Initial Antibiotic Treatment Strategies for Outpatients1
| Empiric Outpatient Regimens | | |
|---|
| | Standard outpatient regimena | Strength of recommendationa |
|---|
| No comorbidities or risk factors for MRSA and Pseudomonas aeruginosaa | Amoxicillin
OR Doxycycline
OR Macrolidec (if local pneumococcal resistance is <25%) | Strong recommendation, moderate quality of
evidence Conditional recommendation, low quality of
evidence Conditional recommendation, moderate quality of
evidence |
| With comorbiditiesb | Option 1
Amoxicillin/clavulanate OR an
oral cephalosporind
PLUS Macrolidec OR doxycycline Option 2
Respiratory fluoroquinolonee | Option 1
Strong recommendation, moderate quality of evidence (Amoxicillin/clavulanate OR cephalosphorind PLUS macrolidec)
Conditional recommendation, low quality of evidence (Amoxicillin/clavulanate OR cephalosporind PLUS doxycycline)
Option 2
Strong recommendation, moderate quality of evidence |
aRisk factors include prior respiratory isolation of MRSA or P. aeruginosa or recent hospitalization AND receipt of parenteral antibiotics (in the last 90 days).
bComorbidities include chronic heart, lung, liver, or renal disease; diabetes mellitus; alcoholism; malignancy; or asplenia.
cAzithromycin, or clarithromycin.
dCefpodoxime, or cefuroxime.
eLevofloxacin, moxifloxacin or gemifloxacin.